In recent years, lead-halide perovskites, as emerging optoelectronic materials, have shown great potential in optoelectronic applications such as solar cells, light-emitting diodes, and lasers. However, the toxicity of lead makes these lead-containing materials pose an environmental problem in practical commercial applications. Therefore, many researchers have put a lot of effort into the design and synthesis of lead-free perovskite materials. At the same time, many new properties of metal halide perovskites have been discovered in the process of developing new lead-free perovskite materials.
Recently, Li's group from China University of Geosciences (Wuhan) and Lin's group from Changchun Institute of Applied Chemistry reported the use of solvents N, N-dimethylformamide (DMF) or N, N-dimethylacetamide (DMAC) induced the phenomenon of fluorescent color change induced by the crystal structure conversion of manganese(II)-based perovskite CsMnCl3(H2O)2. This process exploits the sensitivity of the metal halide perovskite structure to polar solvents and the sensitivity of the luminescence of divalent manganese ions to the coordination environment.
Studies have shown that when CsMnCl3·2H2O is in contact with two polar solvents such as DMF or DMAC, its crystal structure rapidly changes from CsMnCl3·2H2O to Cs3MnCl5 and then to Cs2MnCl4·2H2O, and the luminescence changes from red emission to bright green emission, and then returns to the red emission. The process is fast, easy to observe and has high selectivity, which is beneficial to the application in anti-counterfeiting. The change of the emission color is due to the change of the coordination number of manganese(II) during the phase transition, which leads to the change of the surrounding crystal field intensity. The stronger the crystal field strength, the more red-shifted the luminescence of the divalent manganese ions. Finally, we made test paper and printed patterns by the CsMnCl3·2H2O, all of which have fast response to DMF/DMAC, revealing that this material has potential application prospects in anti-counterfeiting, information encryption and so on.
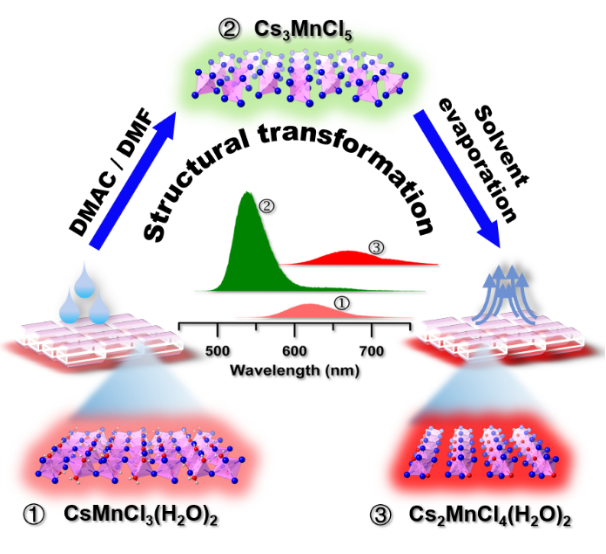
Figure 1. Solvent-induced crystal structure transition and fluorescence color change.
This work demonstrates the solvent-induced crystal-to-crystal structural transition of a lead-free metal halide perovskite and its accompanying change in fluorescence emission. At the same time, this fluorescent color change phenomenon also enables metal halide perovskites to be used in anti-counterfeiting, information encryption, etc., expanding its application range. The article titled "Solvatochromic Photoluminescent Effects in All-Inorganic Manganese(II)-Based Perovskites by Highly Selective Solvent-Induced Crystal-to-Crystal Phase Transformations" was published inAngewandte Chemie International Edition.
This research was supported by the National Natural Science Foundation of China. Prof. Guogang Li from China University of Geosciences (Wuhan) and Prof. Jun Lin and Prof. Ziyong Cheng from Changchun Institute of Applied Chemistry are the corresponding authors of this work, and the first author is Dr. Xiao Hui from Changchun Institute of Applied Chemistry.
Article link: https://onlinelibrary.wiley.com/doi/10.1002/anie. 202012383