Because of complex tumor microenvironment, traditional therapeutic agents frequently suffered from insufficient accumulation in tumor tissues, low cellular internalization efficiency, as well as weak organelle targeting capacity, leading to the unsatisfying therapeutic outcome eventually. To overcome the multiple obstacles in tumor therapy, equipping the therapeutic agents with a series of abilities is a common strategy. By integrating diverse functional moieties like cell selective moiety, cell penetrating moiety, organelle targeting moiety and therapeutic moiety into one system, modular-agent probe (MAP) could be constructed. Nowadays, researchers have tried to optimize the MAPs by exploiting the new modules or increasing the numbers of module, while neglecting the configuration of various modules.
Recently, based on the development of MAPs in the early stage (Angew. Chem. Int. Ed. 2020, 59, 20405-20410;ACS Nano 2020; 14, 14698-14714;Angew. Chem. Int. Ed. 2019, 58, 5049-5053), we alighted on the idea of whether the spatial-arrangement of existing modules, could improve the performance of MAPs. By utilizing a tetraphenylethylene (TPE) derivative with stereochemical structure and dual modifiable end-group sites as small molecule scaffold, two MAPs with same modular agents but different spatial-arrangements are designed.
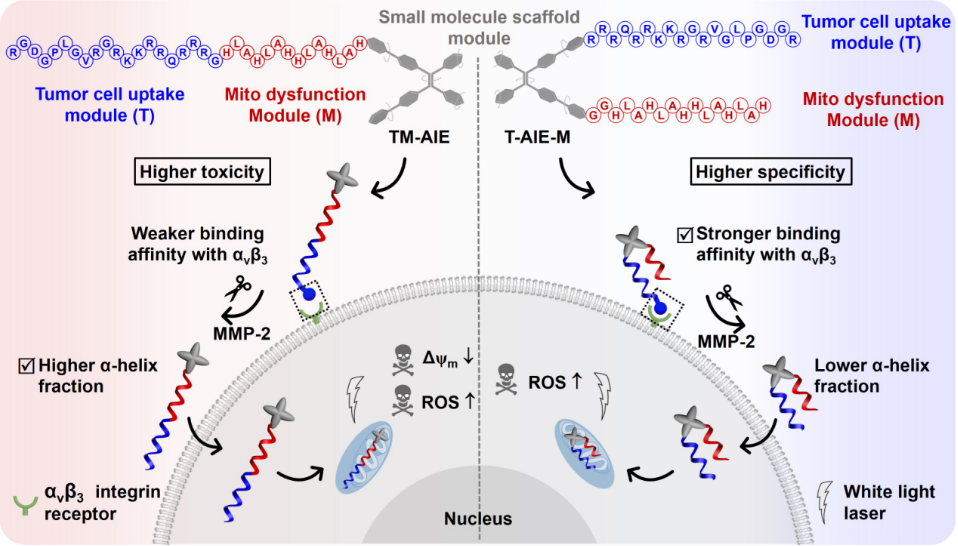
Figure 1. Utilizing small molecule scaffold with dual modifiable sites to connect another two functional modules (T and M), different cancer cell targeting and tumor inhibition performance were achieved.
In this study, the two arrangements of reagent probes (TM-AIE, T-AIE-M) consist of the same three modules. The small molecule scaffold (AIE) is a photosensitive mitochondrial targeted TPE derivative for generating reactive oxygen species (ROS) under irradiation (Figure 1). Peptide module T, aiming at enhancing the intracellular uptake of MAPs in tumor. Peptide module M was contributed to increasing the therapeutic effect through mitochondrial swelling and apoptosis. By comprehensively comparing the intracellular performance of MAPs including specificities (cell selectivity, macropinocytosis enhancement and mitochondrial targeting) and toxicity (ROS generation and mitochondria membrane potential), we found that the T-AIE-M exhibited higher specificities to tumor cells, while TM-AIE displayed superior toxicity. Next, we deeply explore the mechanism that produces the performance difference by comparative analyzing theoretical calculations and cell experimental results. It was found that T-AIE-M with bigger RGD binding angle and higher αvβ3 integrin affinity performed higher specificity, while TM-AIE characterizing higher α-helix fraction displayed superior toxicity.
This significant phenomenon suggests that the potential effects of different spatial arrangements of modules should be properly considered when designing MAPs in the future. The research also opens a window for the renaissance of existing MAPs. The article was published in Angewandte Chemie International Edition under the title " Spatial order of functional modules enabling diverse intracellular performance of fluorescent probes".
This research was supported by the National Key R&D Program of China (2020YFA0211200), the National Natural Science Foundation of China (22090050, 21974128, 21874121, 52003257, 21703210), Hubei Provincial Natural Science Foundation of China (2019CFA043, 2020CFA037). and Prof. Xiaoding Lou form China University of Geosciences (Wuhan) are the corresponding authors of this work, and the first authors are Chong Duan and Jingjing Hu from China University of Geosciences (Wuhan).
Article link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202106195