Target recognition unit is an important core component in biosensing. In the classical biosensing process, the target recognition unit is in the "on" state at the beginning. Once it encounters the target, it directly carries out specific recognition and generates signal output, which makes it difficult to achieve controllable activation and accurate regulation of the process. This process will result in unnecessary interference signal output and consumption of target identification unit when the target identification unit is combined with the target in the process of reaching a specific detection position. Due to the time and space heterogeneity of the target in the actual complex biological samples, it is required to realize the real-time, in-situ and controllable activation of the sensing process with high spatial and temporal resolution on the basis of pursuing the high sensitivity and specificity of the traditional measurement methods, so as to achieve more "accurate" measurement of the target. As a non-invasive control means, photocontrol can achieve "time-space" precise control. Therefore, we designed a photoresponsive functional nucleic acid target recognition unit to photoactivate the biosensing process.
Xia's group from China University of Geosciences (Wuhan) designed photoresponsive functional nucleic acid probe based on the photoresponsive molecule 2-nitrobenzyl as the linker to detect ATP and micRNA in single cell and to explore the interface photoactivated biosensing process.
A kind of o-nitrobenzylphosphate ester hairpin nucleic acid was introduced as a photoresponsive DNA probe for light-activated ATP detection in single living cells. Two methods to spatiotemporally activate the probe in single living cells, the usage of the micrometer-sized optical fiber (about 5 mm) to guide the UV light (l=365 nm) to selectively activate the photoresponsive DNA probe or a two-photon laser scanning microscope to selectively irradiate the photoresponsive DNA probes confined in single living cells irradiation (l=740 nm). ATP aptamer integrated in the activated DNA probes selectively interacted with the target in dictated signal generation. The photoactivated biosensing process enables dictated dual-model ATP detection in single living cells with "Signal-ON" fluorescence signal and "Signal-OFF" electrochemical signal outputs.
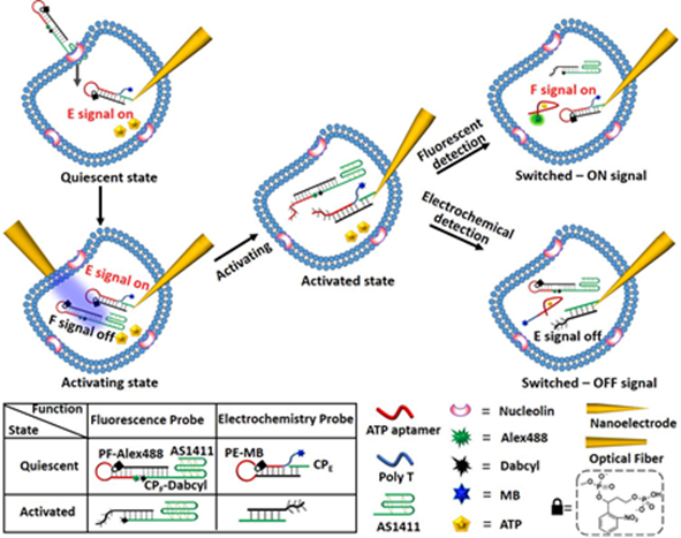
Figure 1. Principle of photoactivated probes for the "Signal-ON"fluorescence signal and "Signal-OFF" electrochemical signal of ATP detection in a single cell.
Furthermore, a developed method is achieved via combing photoactivated nucleic acid displacement reaction with the traditional exonuclease Ⅲ (EXO Ⅲ)-assisted DNA walker based on DNA nanoflares,which enables photocontrollable signal amplification imaging of cancer-related miRNA in single living cells. The developed method was demonstrated as a proof of concept to achieve photoactivated signal amplification imaging of miRNA-21 in single living HeLa cells via selective two-photon irradiation (λ=740 nm) of single living HeLa cells by using confocal microscopy equipped with a femtosecond laser.

Figure 2. Schematic overview of UV light-photoactivated DNA walker based on DNA nanoflares in Hela cells for dictated signal amplification miRNA imaging.
Inspired by previous work, we introduce the photolithographic patterning or two-photon laser scanning confocal microscopy patterning of a series of o-nitrobenzylphosphate ester nucleic acid-based polyacrylamide hydrogel films generating periodically-spaced circular patterned domains surrounded by continuous hydrogel matrices. The patterning processes lead to guided modulated stiffness differences between the patterned domains and the surrounding hydrogel matrices, and to
the selective functionalization of sub-regions of the films with nucleic acid anchoring tethers. HeLa cells are deposited on the circularly-shaped domains functionalized with the MUC-1 aptamers. Initiation of the hybridization chain reaction by nucleic acid tethers associated with the continuous hydrogel matrix results in stress-induced ordered orthogonal shape-changes on the patterned domains, leading to ordered shapes of cell aggregates bound to the patterns.
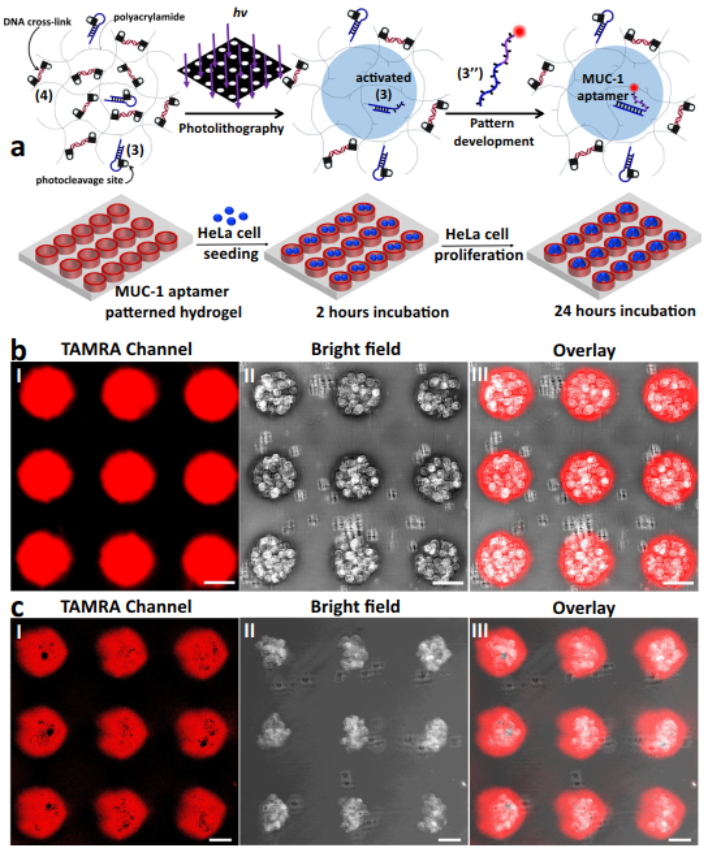
Figure 3. Patterning of a photoresponsive hydrogel for the selective binding and proliferation of HeLa cells in the confined patterned domains.
These research apply photoresponsive nucleic acids in biosensing process to realize “more accurate” detection in single cell with high spatiotemporal resolution, and to understand the spatiotemporal effects of surface topographies and modulated stiffness and anisotropic stress of hydrogels on cell growth. These articles were published in Analytical Chemistry, Analytical Chemistry, Nature Communication Edition under the title "Photoactivated Biosensing Process for Dictated ATP Detection in Single Living Cells", "Photoactivated DNA Walker Based on DNA Nanoflares for Signal-Amplified MicroRNA Imaging in Single Living Cells", "Photoactivated DNA Walker Based on DNA Nanoflares for Signal-Amplified MicroRNA Imaging in Single Living Cells", "Spatiotemporal patterning of photoresponsive DNA-based hydrogels to tune local cell responses".
These researches were supported by the National Natural Science Foundation of China (21974127, 22090050, and 21874121), the National Key Research and Development Program of China (2018YFE0206900), Hubei Provincial Natural Science Foundation of China (2020CFA037), and Zhejiang Provincial Natural Science Foundation of China under grant nosLD21B050001 and LY20B050001.
Article link: https://doi.org/10.1021/acs.analchem.1c02049
https://doi.org/10.1021/acs.analchem.1c04505
https://doi.org/10.1038/s41467-021-22645-8