The electrochemical aptamer-based (E-AB) sensorshave the advantages of rapid detection, high signal sensitivity and direct detection of complex samples without pretreatment, providing a good platform for real-time detection of drugs in blood samples or organisms. Due to the complexity of the blood sample composition (including blood cells, platelets, and a wide variety of protein molecules, etc.), the E-AB sensorsneed to overcome the least two scientific problems, low sensor-to-sensor reproducibility and often-severe baseline drift issues when deployed directly in whole blood.
Recently,Xia/Li's group from China University of Geosciences (Wuhan)focused on the E-AB biosensor detection in blood samples of the existence ofdifferences in different sensors and baseline unstable, developing a calibration-free “O-N” approach to detection target based on the hybridization chain reaction (HCR) amplification strategy. This approach employs two types of redox reporters, denoted as “One reporter” and “N reporters”, with the former attached on the capture DNA and the latter on H1 and H2 strands. By optimizing the attachment sites of these reporters onto DNA strands, we demonstrate a significantly enhanced sensitivity of such sensor platform by four orders of magnitude, achieving accurate, calibration-free measurement of nucleic acids including ctDNA directly in undiluted whole blood without the requirement to calibrate each individual sensor.Those twotypes of redox reporters (MB and Fc) sensors decrease thedifference between two sensors, improvement of sensors reproducibility (Figure 1,Anal. Chem. 2021, 93, 8354−8361).
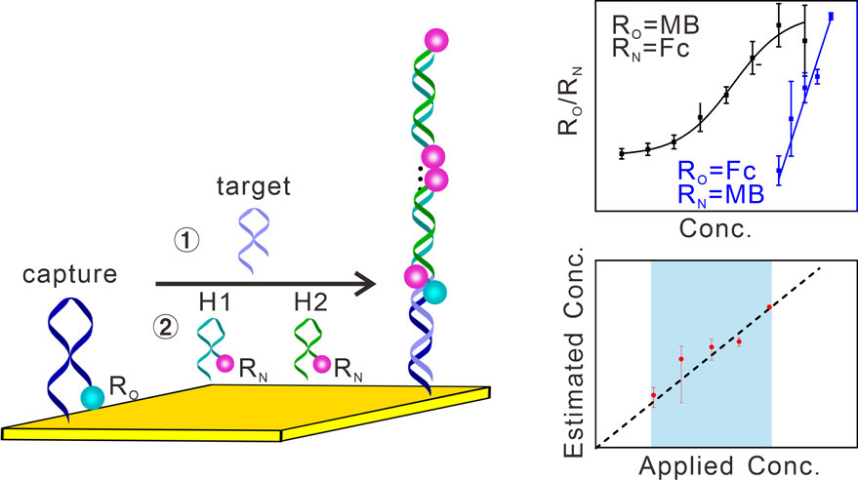
Figure 1: Hybridization Chain Reaction-Amplified Electrochemical DNA-Based Sensors Enable Calibration-Free Measurements of Nucleic Acids Directly in Whole Blood
In the meanwhile, Hui Li’s group bionic membrane system was introduced into the E-AB sensor platform to improve the anti-adsorption ability of electrode surface, and solve the problem of unstable test baseline in complex system. Typically, E-AB sensors are comprised of three main components (Figure 2): (1) DNA or RNA aptamer, a class of functional oligonucleotides that bind a specific analyte and can be artificially selected via high-throughput, in-vitro methodologies; (2) redox reporter, here methylene blue, which is covalently attached to the aptamer and serves as a signal indicator; (3) self-assembled monolayers (SAMs), which serve as a molecular blocking layer to improve the signals and antifouling properties. Alternatively, they recently demonstrated the use of a biomimetic, antifouling monolayer consistingphorsphorycholine (PC) moieties to greatly improve the baseline stability of in-vivo electrochemical sensors.

Figure 2: Exploring End-Group Effect of Alkanethiol Self-Assembled Monolayers on Electrochemical Aptamer-Based Sensors in Biological Fluids
SAMs featuring zwitterionic structures arewell known for their profound antifoulingproperties. Li’s groupcarefully designed a series ofalkanethiol molecules featuringnoncharge,monocharge, or zwitterionic moieties, with each subtype containing two molecules, including nonchargedoligoethylene glycol (OEG);monocharged ones, e.g., trimethylammonium chloride (AC) and sulfopropylmethacrylate potassium (SP); and zwitterionic ones, e.g.,(dimethylammonio)propane sulfonate (AP). With this, they achieved a good modulation of surface properties including their wettability and antifouling properties in different test environments (whole blood, urine, sweat). This provides, in retrospect, a great opportunity to modulate the signaling performance of E-AB sensors when incorporating these SAMs into the sensor platform as a blocking layer, achieving adjustable dissociation constants spanning over 2 orders of magnitudes. This work was published onAnal. Chem. 2021, 93, 14, 5849–5855.
This research work was supported by the National Natural Science Foundation of China. Prof. Hui Li is the corresponding authors of both publications and the first author is theProf. Shaoguang Li.
Articlelink: https://pubs.acs.org/doi/10.1021/acs.analchem.1c01436
https://pubs.acs.org/doi/10.1021/acs.analchem.1c00085